Acute myeloid leukemias (AMLs) are often driven by genomic translocations, of which t(8;21) is the most frequent. The resulting gene fusion RUNX1::RUNX1T has been shown in cell line and transgenic expression models to impede cell differentiation and promote leukemic self-renewal. However, it has remained unclear if and how RUNX1::RUNX1T1 influences leukemic propagation in patient-derived AML cells.
To address the role of RUNX1::RUNX1T1 in the differentiation status and heterogeneity of patient-derived leukemic blasts, we developed an siRNA lipid nanoparticle (LNP) delivery system for the fusion transcript knockdown (Issa et al, Leukemia 2023). For efficient delivery to primary cells, the LNPs were functionalized with a Leu-Asp-Val (LDV) tripeptide binding the Very Late antigen-4 receptor on leukocytes. Primary AML cells were cultured and expanded for several weeks using a serum-free co-culture system with human bone marrow-derived mesenchymal stromal cells (MSCs) (Swart et al, Pharmaceutics 2023). Treatment of t(8;21) patient-derived xenograft (PDX) and primary bone marrow aspirates depleted RUNX1::RUNX1T1 transcript and protein, as determined by RT-qPCR and western blotting.
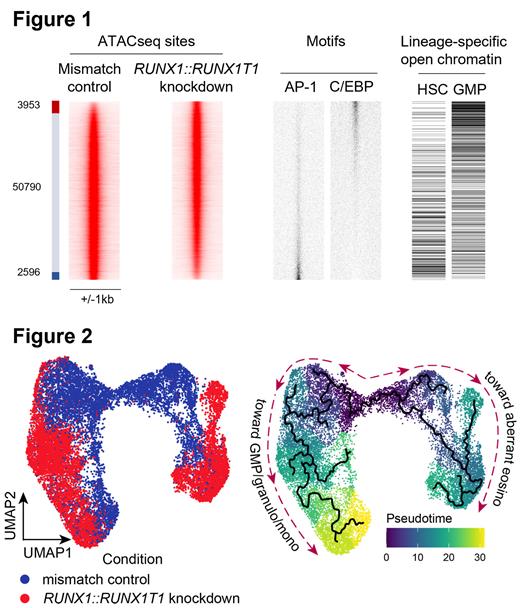
We unraveled the transcriptional repercussions of RUNX1::RUNX1T1 knockdown using bulk RNAseq and ATACseq in a PDX and identified 1068 up- and 1521 downregulated genes upon RUNX1::RUNX1T1 knockdown, as compared to mismatched siRNA-treated cells (padj < 0.001, log2FC > 2). Gene set enrichment analysis revealed loss of self-renewal potential and enhanced myeloid differentiation. Similarly, ATACseq revealed an increased GMP/monocyte-like chromatin accessibility and a decreased HCS/MPP-like one upon RUNX1::RUNX1T1 knockdown (Figure 1). Surprisingly and in contrast to previous cell line results, RUNX1::RUNX1T1 depletion correlated with increased cell cycle and DNA repair signatures. These combined data demonstrate that RUNX1::RUNX1T1 perturbance promotes differentiation of leukemic stem cells towards granulocytic and monocytic lineages. Furthermore, they suggest that the initial stages of differentiation are linked to increased proliferation in line with the increased proliferation capacity of GMPs compared to more immature progenitor states.
To investigate the impact of RUNX1::RUNX1T1 on leukemic blast populations at greater granularity, we knocked RUNX1::RUNX1T1 down in 2 primary bone marrow aspirates and 1 PDX and performed single-cell (sc) RNAseq. Notably, principal component analysis revealed that loss of RUNX1::RUNX1T1 shifted all leukemic populations, while hardly affecting normal hematopoietic cells or MSCs (Figure 2). In all samples, pseudotime analysis showed a more differentiated state of cells upon RUNX1::RUNX1T1 depletion. For cell type annotation, we projected our datasets onto a reference normal bone marrow dataset (van Galen et al, Cell 2019). This projection confirmed differentiation toward granulocyte-monocyte progenitors (GMPs), granulocytes and monocytes. Strikingly, the confidence of cell type prediction according to the reference normal bone marrow was significantly and substantially higher upon RUNX1::RUNX1T1 depletion than in cells with unimpaired fusion transcript level, suggesting restoration of normal myeloid differentiation.
In addition to that, primary RUNX1::RUNX1T1 AML samples also contained a new, distinct cell subpopulation that emerged upon RUNX1::RUNX1T1 depletion. That subpopulation could not be annotated as resembling any normal bone marrow cell type. However, it expressed eosinophil marker genes such as RNASE3 and EPX. We, therefore, labeled this cell subpopulation as aberrant eosinophilic. This reveals RUNX1::RUNX1T1's role in disrupting differentiation towards multiple lineages.
Our work illustrates a strong dependency of the differentiation potential of t(8;21) AML cells on their driver gene RUNX1::RUNX1T1 in an ex vivo setting with PDX or primary cells. We demonstrate that RUNX1::RUNX1T1 hijacks more than one differentiation trajectory of the AML blasts. Our findings also highlight siRNA-loaded LNPs as an innovative therapeutic approach for fusion gene-driven AML by directly targeting the fusion gene as the leukemic driver.
Disclosures
Schiffelers:Excytex: Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees; Anjarium BioSciences: Membership on an entity's Board of Directors or advisory committees; SentryX: Membership on an entity's Board of Directors or advisory committees; NanoCell Therapeutics: Current Employment. Heidenreich:Roche: Research Funding; Syndax: Research Funding.